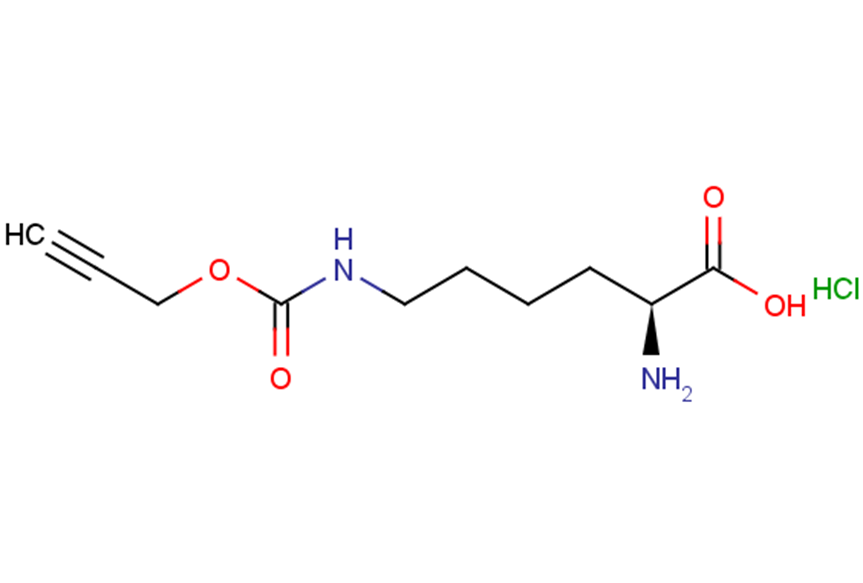
N-ε-propargyloxycarbonyl-L-lysine hydrochloride
CAS No. 1428330-91-9
N-ε-propargyloxycarbonyl-L-lysine hydrochloride( H-?L-?Lys(Poc)?-?OH (hydrochloride),N6-((Prop-2-yn-1-yloxy)carbonyl)-L-lysine hydrochloride )
Catalog No. M22480 CAS No. 1428330-91-9
N-ε-propargyloxycarbonyl-L-lysine hydrochloride is a modified amino acid for cancer therapy development.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 10MG | 35 | In Stock |


|
| 25MG | 56 | In Stock |


|
| 50MG | 80 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameN-ε-propargyloxycarbonyl-L-lysine hydrochloride
-
NoteResearch use only, not for human use.
-
Brief DescriptionN-ε-propargyloxycarbonyl-L-lysine hydrochloride is a modified amino acid for cancer therapy development.
-
DescriptionN-ε-propargyloxycarbonyl-L-lysine hydrochloride is a modified amino acid for cancer therapy development.
-
In VitroLabeling of N-ε-propargyloxycarbonyl-L-lysine (H-L-Lys(Poc)-OH) hydrochloride-carrying cellular proteins, such as Sec61β, Htt74Q and the histone H3 variant H3.3, with a sensitive Raman tag by click chemistry for molecular hyperspectral SRS imaging.
-
In Vivo——
-
SynonymsH-?L-?Lys(Poc)?-?OH (hydrochloride),N6-((Prop-2-yn-1-yloxy)carbonyl)-L-lysine hydrochloride
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1428330-91-9
-
Formula Weight264.71
-
Molecular FormulaC10H17ClN2O4
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (377.77 mM)
-
SMILESCl.N[C@@H](CCCCNC(=O)OCC#C)C(O)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. Enke HEIKE, et al. Modified microcystins and nodularins
molnova catalog



related products
-
24-epi-24-O-acetyl-7...
The roots of Cimicifuga foetida L.
-
L-Dithiothreitol
L-Dithiothreitol is a chiral bidentate dithiol with two stereogenic centers. It may be used in chiroptical response research.
-
1-Methylindazole-3-c...
It is an excellent solvent with proper boiling point.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


